Hanta Virus
Hantavirus pulmonary syndrome (HPS) was first described in 1993 in the southwestern United States. HPS is a respiratory illness associated with the inhalation of aerosolized rodent excreta (urine and feces) contaminated by Hantavirus particles. Four Hantavirus species have been implicated as etiological agents of HPS in North America (1). One species, Sin Nombre virus, has been associated with the largest proportion of HPS cases. Its primary reservoir is the deer mouse, Peromyscus maniculatus. Person-to-person transmission of HPS has not been documented in North America.
Epidemiology in Canada
Although HPS was made a nationally notifiable disease as of 1 January
2000, provincial and territorial public-health authorities have
previously reported confirmed cases. The first case of HPS recognized
in Canada during active surveillance was in 1994 in British Columbia.
Subsequently cases have been identified retrospectively with the
earliest case dating back to 1989 in Alberta. As of 31 December 1999,
32 laboratory-confirmed cases have been reported in Canada with a case
fatality rate of 38% (12/32). The average age of HPS cases has been 39
years (range: 15 to 62 years). The majority of cases (19/32, 60%) have
been male.
Cases have only been reported from western Canada. The majority of
these have been reported from Alberta (20) with most originating in
clusters southeast and northwest of Edmonton. Remaining cases have
been+ reported from British Columbia (6), Saskatchewan (5), and
Manitoba (1). The geographical distribution of Canadian HPS cases are
similar to that in the United States where less densely populated
western regions have experienced the greatest burden of disease.
Illness resulting from the infection is usually severe and occurs
within 45 days from the exposure to the virus. It begins with acute
flu-like symptoms including fever, muscle pain, cough, headache,
nausea, vomiting and shortness of breath followed by fluid in the lungs
and hypotension (inadequate blood pressure to pump blood to tissues and
organs). Death results in greater than 60% of all cases and usually
occurs within nine days of the onset of symptoms.
Orientation
Persons in potentially high-risk settings should be thoroughly informed
about the symptoms of this disease and given detailed guidance on
preventative measures.
Cautionary Note
You should contact your physician immediately if you experience fever,
muscle pain, cough, headache, nausea, vomiting, shortness of breath or
any respiratory illness within 45 days of potentially being exposed to
the Hanta Virus (i.e. working, living, camping in areas where the
potential exists to be exposed to rodents, rodent feces, urine, etc.).
Advise your doctor of the potential occupational risk and have him/her
contact the local health authorities regarding Hanta Virus-associated
illness, testing and treatment.
Personal Protective Equipment (PPE) - General Description
The following personal protective equipment shall be worn whenever dealing with rodents or rodent feces, urine, saliva or blood.
- Coveralls or surgeon's gown (preferably disposable).
- Gloves
- Rubber - thick (neoprene, latex, or butyl) for removing dead rodents from traps. Gloves should be disinfected with 5% Lysol or 10% bleach before removing and hands should be thoroughly washed with soap and water after gloves are removed.
- Latex - 2 pairs should be worn when removing organs or obtaining blood. Treat gloves and hands as above and discard gloves after use.
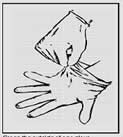
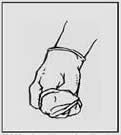
1. Grasp the outside of one glove. 2. Hold the glove with your hand. 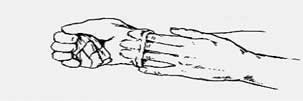
3. Insert your fingers on the inside of the glove. 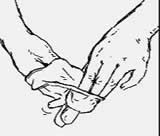
- Leather
trapping gloves worn over latex gloves should be used to remove live
animals from traps. Unless badly contaminated, gloves can be
disinfected with 5% Lysol or 10% bleach and air dried for subsequent
use. Treat latex gloves as above.
Note: Leather gloves do not provide protection from the virus, only from bites scratches etc.
- Eye Protection
Protective goggles (Chemical splash type) are to be worn to prevent contact with eye tissue and tear ducts. - Respirator
a half face, dual cartridge type respirator or a powered air purifying respirator (PAPR) is acceptable. Respirators must be equipped with HEPA (high efficiency particulate aerosol) filters. Other types of cartridges are not acceptable. The respirator must be properly fit tested prior to each use with either irritant smoke or isoamyl acetate. Instruction and training on the proper fit, cleaning and maintenance of the respirator must be given (contact the OH&S department).
Prior to each use a negative or positive pressure test must be performed (as demonstrated during the fit test.) When breathing becomes difficult it indicates that the HEPA filter is plugged. It should be removed with gloved hands, bagged and treated as biohazardous waste (place in autoclave bag for autoclaving). The mask (not the filters) should be decontaminated with 70% isopropyl alcohol after each use and stored in a clean plastic bag, separate from the HEPA filter cartridges. The face (front) of the filter cartridge should be taped over with duct tape to prevent any materials collected on the filter from contaminating the storage bag or the inside of the filter. The filters should then be placed in a plastic bag if intended for re-use or into an autoclave bag if the cartridges are to be autoclaved and discarded. - Rubber boots or boot covers
Rubber boots or disposable synthetic boot covers are to be used. Disinfect and dispose of in the same manner as with gloves. All PPE is considered contaminated after use and must be either disinfected and air dried, or washed with hot water, bleach (powdered bleach if clothing is not colour fast) and detergent, then dried in a hot dryer (may also be air dried in the sun). If no laundry facilities are readily available, clothing should be immersed in liquid disinfectant until they can be washed. When laundering clothing, use gloves to place the items into the washing machine. Wash gloved hands in a disinfectant then remove gloves and wash hands in soap and water. If that is not possible then the clothing should be placed in a bucket of disinfectant. If articles are to be discarded, they should be soaked with disinfectant and double bagged for proper disposal later (autoclave bag or regular trash).
Waste Disposal
All potentially infective waste, including respirator cartridges,
cleaning materials, etc., from operations are to be double bagged and
labelled as an infectious or biomedical hazard. These materials will
need to be autoclaved prior to disposal in accordance with requirements
for management of biohazardous waste because they can not be cleaned
before disposal. Items which can be cleaned before disposal, e.g.,
gloves, coveralls, boots and hood can be disposed of in the garbage as
described below.
Protocols for Rodent Studies and Field Trips
Guidelines for Studies involving Rodents
- Worker's must wear protective clothing.
- A supply of water, disinfectant (5% Lysol or 10% bleach) and soap must be available for worker's to clean skin and clothing with. Lysol and bleach should not be used on the skin.
- Sherman live-capture traps are recommended over a wire mesh trap as the solid design may offer some protection against aerosols. Traps can be disinfected by placing in a 5 gallon container of disinfectant for 10 minutes. Dirt and fecal material on traps should be scrubbed off with a toilet brush while the traps are in the disinfectant. Rubber gloves should be worn over the latex to prevent ripping.
- Live rodents removed from the traps for study (take care not to get urine or feces on skin or clothing) should be placed in double plastic bags and kept out of the sun until they can be anaesthetized. Conversely the trap and rodent may be placed in a double plastic bag containing gauze soaked with an inhalant anesthetic.
- If the animals are processed outside, the area should be away from other humans and animals. When removing animals from traps, workers should sit with their back to the wind with the animal downwind.
Camping or Hiking
Travel to wilderness locations need not be restricted but it is advisable to follow certain precautions.
- Avoid coming into contact with rodents and rodent burrows or disturbing nests.
- Do not use cabins or other enclosures unless they have been thoroughly cleaned and disinfected (5% Lysol or 10% bleach).
- Do not pitch tents or set up camp where there is evidence of rodent feces or burrows or where rodents may build nests (garbage or wood piles).
- If possible do not sleep on bare ground. Use a cot that is at least 30 cm above the ground and use tents with floors.
- Keep food and water in rodent proof containers
- All trash and garbage should be burned (if permissible) then bury the ashes or discard in covered garbage containers (recreation site, Provincial Camp site etc.)
- Use only bottled water or water that has
been disinfected by boiling, chlorination or iodination for drinking,
washing dishes and brushing teeth. Filtration is not adequate for the
decontamination of water that may contain this virus.
Use one of the following methods to decontaminate water:- Chlorination: 1-2 drops of fresh household bleach (5%) per litre of water (if water is clear). If water is cloudy use 2-4 drops of bleach. Thoroughly mix the bleach and water in a clean container and allow to stand for not less than 20 minutes. If water is very cold, double the standing time.
- Boiling: 5 minutes should be sufficient. This is the easiest and most effective method as it kills all known pathogens.
- Iodination: 5 drops of iodine (2% tincture) per
litre of water (if water is clear). If water is cloudy use 10 drops of
iodine. Thoroughly mix in a clean container and let stand for at least
30 minutes. If water is very cold, the effectiveness of this method
will decrease so let stand for an hour or more.
Do not drink water treated with iodine for more than a few days at a time.
Additional Information
Since the exact vector for this virus in BC is unconfirmed, the
Occupational Health and Safety Department will be maintaining
communications with the Centres for Disease Control in BC and Atlanta,
Georgia. Any persons at TRU planning any research involving rodents or
wishing other information on this virus are advised to contact the TRU
Occupational Health and Safety.
